Depo-Provera
Depo-Provera (medroxyprogesterone acetate) is a prescription birth control shot. It’s also available in generic form. Mild side effects typically subside in 2 to 3 months and include nausea and headaches. The drug has a black box warning for bone mineral density loss.
What is Depo-Provera?
Depo-Provera, sometimes called the Depo shot or the birth control shot, is a prescription medication indicated to prevent pregnancy. Its active ingredient is medroxyprogesterone acetate, and it’s typically administered by a medical provider in a clinic or office.
The birth control shot is also available in generic and as DepoSubQ-Provera. DepoSubQ is indicated to prevent pregnancy and to reduce endometrial pain.
Patients get one shot every three months to prevent pregnancy, and Depo-Provera is about 96% effective at preventing pregnancy.
How Does Depo-Provera Work?
The active ingredient in Depo-Provera, medroxyprogesterone acetate, contains the hormone progestin, and it works by preventing ovulation to prevent pregnancy.
“You don’t have to remember to take anything every day, and it lasts for 13 weeks (approximately 3 months). It’s also highly effective and reliable at preventing pregnancy in the near future,” oncology clinical pharmacist and clinical trial scientist Dazhi Liu said about Depo-Provera’s pros.
Patients receive the Depo-Provera shot from their health care provider into the arm or buttocks.
“You don’t have to remember to take anything every day, and it lasts for 13 weeks (approximately 3 months). It’s also highly effective and reliable at preventing pregnancy in the near future.”
Patients who want to try and give themselves the shot will likely be prescribed a second formula, DepoSubQ-Provera 104 mg. This version of the shot is FDA-approved to prevent pregnancy and pain from endometriosis. Medical providers inject it into the abdomen or upper thigh, and patients can be trained to do it themselves at home.
DepoSubQ also helps women with endometrial pain. It works by suppressing new endometrial tissue growth. Another potential benefit to DepoSubQ is that it may help prevent endometrial cancer.
| Dosage and Administration for Depo-Provera and DepoSubQ-Provera | ||
|---|---|---|
| Formula | Depo Provera | Depo-SubQ-Provera |
| Dose Schedule | 1 injection every 12 weeks | 1 injection every 12 to 14 weeks |
| FDA Approval | Birth Control | Birth Control or Endometrial Pain |
| Injection Administered By | Medical Provider | Medical Provider or Patient |
| Injection Administration Site | Arm or Buttocks | Abdomen or Upper Thigh |
| Strength | 150 mg | 104 mg |
Only take Depo-Provera and DepoSubQ-Provera as directed, and don’t change your dose or dosing schedule without talking to your doctor.
Depo-Provera Side Effects
In clinical trials, common Depo-Provera side effects were reported as those that occurred in more than 5% of study participants. Trial data came from two clinical trials with over 3,000 women treated for up to seven years.
The drug’s label also reported on potential serious side effects. These are rare, but they may be medical emergencies. Make sure to tell your doctor about any side effects.
Common Side Effects
Common Depo-Provera side effects are usually mild and go away within two to three months. If any side effect is bothersome or gets in the way of your daily activities, make sure to tell your medical provider right away. Another birth control option might be more effective and more comfortable for you.
- Weight gain
- Not getting a period
- Nervousness
- Irregular bleeding
- Headache
- Dizziness
- Decreased libido
- Abdominal pain or discomfort
Side effects that led to women dropping out of the trial are amenorrhea, bleeding and weight gain.
Serious Side Effects
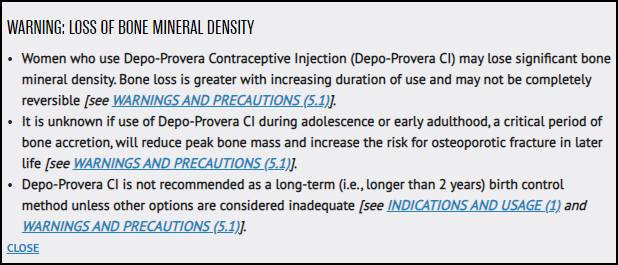
Depo-Provera may lead to serious side effects such as bone density loss, blood clots and allergic reactions.
All formulas of Depo-Provera carry a boxed warning for loss of bone mineral density. Boxed warnings are reserved for the most serious side effects. In this case, the bone mineral density loss caused by Depo-Provera may be irreversible.
- Allergic reactions (swelling of face, neck or tongue)
- Blood clots
- Slight increase in breast cancer risk
- Ectopic pregnancy
- Depression
- Risk of hyperglycemia in patient with diabetes
If you experience symptoms of a blood clot, allergic reactions or ectopic pregnancy, contact your medical provider right away. If you can’t reach your medical provider, seek emergency medical attention.
Depo-Provera Brain Tumor Risk
According to researchers, Depo-Provera users had a 5.6-fold increase in the risk of developing meningioma, a noncancerous brain tumor, with long-term use. These findings appeared in a March 2024 study published in BMJ.
It’s important to note that this study is the first of its kind and researchers say more studies are needed to better understand the risk.
After the study came out, lawyers began investigating Depo-Provera lawsuits on behalf of users who took. If you are concerned about meningioma risk, make sure to discuss your risk with your doctor.
Depo-Provera Isn’t for Everyone
While Depo-Provera is very effective at preventing pregnancy, some people shouldn’t use it if they have certain pre-existing conditions or health issues.
- Are allergic to medroxyprogesterone acetate or any ingredients in the shot
- Suffered from blood clots in your lungs, arms or legs
- Have any history or risks for breast cancer
- Have liver disease or liver problems
- Have unexplained vaginal bleeding
- Suffered a stroke
Before choosing Depo-Provera, make sure you tell your medical provider about any health conditions or allergies.
Depo-Provera Pros and Cons
According to Dr. Dazhi Liu, Depo-Provera has pros and cons, such as ease of use and how long it takes to get your fertility back after stopping the drug. These are just some of the things to consider if you are thinking about taking the birth control shot.
Dr. Liu has provided a list of the most common pros and cons listed below.
- You don’t have to remember to take anything every day – last for 13 weeks (approximately 3 months).
- Highly effective and reliable at preventing pregnancy in the near future.
- Doesn’t interfere with having sex.
- Most women will not have periods while on Depo-Provera.
- Reduces the risk of endometrial cancer (cancer of the lining of the womb) by 80%.
- May help women who have heavy or painful periods.
- Does not protect against STIs, so it’s important to use condoms when you have sex.
- May cause irregular bleeding, no periods or occasional heavy bleeding. This is more common on first starting to use Depo-Provera and often improves with time.
- Once you have had an injection of Depo-Provera, it lasts at least 13 weeks which can be a nuisance if you experience a side effect.
- Your periods and fertility take an average of 6 months to return after stopping the injection.
Make sure to discuss your concerns with your doctor. Birth control is most effective when you can adhere to your dosing schedule, and the right option for you may be the one that allows you to be the most compliant with the least number of side effects.
It’s important to note that the drug’s label doesn’t recommend Depo-Provera for long-term use of more than two years.
5 Cited Research Articles
Consumernotice.org adheres to the highest ethical standards for content production and references only credible sources of information, including government reports, interviews with experts, highly regarded nonprofit organizations, peer-reviewed journals, court records and academic organizations. You can learn more about our dedication to relevance, accuracy and transparency by reading our editorial policy.
- DailyMed. (2024, July 26). DEPO-PROVERA- medroxyprogesterone acetate injection, suspension prescribing information. Retrieved from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=199cf13e-0859-4a73-9b45-e700d0cd1049#S5.3
- DailyMed. (2024, July 26). DEPO-SUBQ PROVERA- medroxyprogesterone acetate injection, suspension prescribing information. Retrieved from https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=390087a6-f3c3-4f0b-a930-79acf412f153&type=display
- Roland, N. et al. (2024, March 27). Use of progestogens and the risk of intracranial meningioma: national case-control study. Retrieved from https://www.bmj.com/content/384/bmj-2023-078078.long
- Sample, I. (2024, March 27). Hormone medication could increase risk of brain tumours, French study finds. Retrieved from https://www.theguardian.com/society/2024/mar/27/hormone-medication-brain-tumours-risk-progestogens-study
- Planned Parenthood. (n.d.). Birth Control Shot. Retrieved from https://www.plannedparenthood.org/learn/birth-control/birth-control-shot
Calling this number connects you with a Consumer Notice, LLC representative. We will direct you to one of our trusted legal partners for a free case review.
Consumer Notice, LLC's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
866-934-1922
