Depo-Provera Side Effects
Rare but serious Depo-Provera side effects include loss of bone mineral density, increased risk of breast cancer and blood. A 2024 study found a potential increased risk of nonmalignant brain tumors called meningioma in Depo-Provera users. Common Depo-Provera side effects include weight gain and irregular vaginal bleeding.
Common Depo-Provera Side Effects
The most common Depo-Provera side effects found in clinical trials occurred in more than 5% of test subjects. Other mild side effects occurred at a lower frequency of about 1 to 5%. In adverse event clinical trials, over 3,000 women treated with Depo-Provera were followed for up to seven years.
- Abdominal pain or discomfort (11.2%)
- Acne (1.2%)
- Alopecia (1.1%)
- Arthralgia (1%)
- Backache (2.2%)
- Bloating (2.3%)
- Breast pain (2.8%)
- Decreased sex drive (5.5%)
- Depression (1.5%)
- Dizziness (5.6%)
- Dysmenorrhea (1.7%)
- Edema (2.2%)
- Fatigue (4.2%)
- Headache (16.5%)
- Hot flashes (1%)
- Insomnia (1.0%)
- Irregular bleeding (57.3% at 12 months, 32.1% at 24 months)
- Leukorrhea (2.9%)
- Meg cramps (3.7%)
- Nausea (3.3%)
- Nervousness (10.8%)
- Not getting a period (55% at 12 months, 68% at 24 months)
- Rash (1.1%)
- Vaginitis (1.2%)
- Weight gain, more than 10 pounds after two years (37.7%)
The median study duration was 13 months, and 58% of patients remained in the study after 13 months and 34% after 24 months. The most common reasons for dropping out of the study were bleeding, amenorrhea and weight gain.
After stopping Depo-Provera, women who want to get pregnant may need to wait several months for their fertility to return. However, women who don’t want to get pregnant after stopping Depo-Provera should still use another method of contraception to avoid pregnancy.
Serious Depo-Provera Side Effects
Rare but serious side effects may occur with Depo-Provera, including breast cancer, bone density loss and increased risk of blood clots.
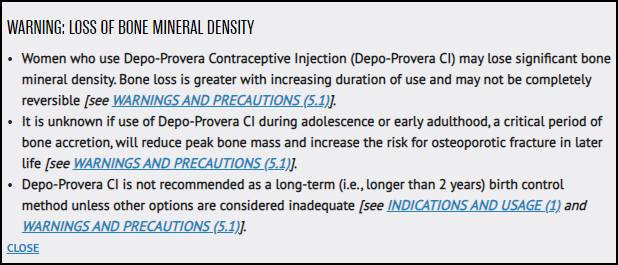
Pfizer added a black box warning to the drug’s prescribing label for loss of bone mineral density. A black box warning is the FDA’s strongest warning. Make sure to discuss any potential risks with your doctor.
Breast Cancer Risk
According to the drug’s label, Depo-Provera may increase the risk of breast cancer. Of the studies conducted, three studies suggest a slightly increased risk of breast cancer among the birth control shot users.
increased risk was particularly seen in women who used Depo-Provera for 12 months or longer.
Bone Mineral Density Loss
Depo-Provera is associated with significant loss of bone mineral density because it lowers estrogen levels. Bones may be at increased risk of fracture in adolescents and young adults because bone is still forming at this age.
Women who used Depo-Provera for more than two years still had not fully recovered bone loss after five years post Depo-Provera treatment. Depo-Provera shouldn’t be used for more than two years unless it’s the only effective option for a patient.
Blood Clots
Blood clots are a known risk of Depo-Provera and other hormonal contraceptives. While the birth control shot hasn’t been proven to be associated with the induction of thrombotic or thromboembolic disorders, blood clots have been reported.
Brain Tumor Risk with Depo-Provera
A March 2024 study published in BMJ by French researchers found that Depo-Provera increased the risk of developing meningioma, a noncancerous brain tumor, by 5.6-fold. But they only found an increase in risk with long-term use. The study is the first of its kind and more research is needed to learn more about the risk.
These types of tumors are not typically cancerous, but the mass can grow and affect surrounding brain tissue and impinge on nerve or blood vessels causing serious issues. When this happens, doctors may perform brain surgery which could be risky.
“Surgical resection, which is the surgical removal of a tumor, is the primary choice for symptomatic meningiomas or large tumors that are anticipated to cause symptoms soon.”
“Surgical resection, which is the surgical removal of a tumor, is the primary choice for symptomatic meningiomas or large tumors that are anticipated to cause symptoms soon. Total removal (also called gross total resection, or GTR) can cure the majority (about 70% to 80%) of people with meningiomas. The goal of surgery is maximum, safe removal,” according to Drugwatch expert and oncology clinical pharmacy specialist, Dazhi Liu.
After the study was published, lawyers began investigating Depo-Provera lawsuits for plaintiffs who developed meningioma after using the brand name or generic form of the drug.
When Should You Call a Doctor?
While common side effects of Depo-Provera are typically mild and should go away after about 2-3 months as the body gets used to the medication, some side effects are very serious and are medical emergencies.
- Signs of a potential allergic reaction: Swelling in the face, mouth, tongue or neck
- Hives or other type of rash
- Signs of jaundice: Yellowing of skin or eyes
- Severe lower abdominal tenderness or pain
- Breathing difficulties
- Persistent pain, bleeding at the injection site or pus
- Signs of a possible blood clot in the eye: Sudden blindness, partial or complete
- Signs of a possible stroke: Sudden severe headache, dizziness, vomiting, fainting, problems with your eyesight, weakness, speech problems, numbness in an arm or leg
- Signs of a potential clot in the leg: Severe pain or swelling in the calf
- Symptoms of a potential blood clot in the lungs: Chest pain, sudden shortness of breath, coughing up blood
- Vaginal bleeding that is unusually heavy
A person’s state of health, genetics and other health conditions may affect the severity or duration of side effects. Make sure to speak with a health care professional if any symptoms become bothersome or interfere with daily life.
4 Cited Research Articles
Consumernotice.org adheres to the highest ethical standards for content production and references only credible sources of information, including government reports, interviews with experts, highly regarded nonprofit organizations, peer-reviewed journals, court records and academic organizations. You can learn more about our dedication to relevance, accuracy and transparency by reading our editorial policy.
- DailyMed. (2024, July 26). DEPO-PROVERA- medroxyprogesterone acetate injection, suspension. Retrieved from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=199cf13e-0859-4a73-9b45-e700d0cd1049#S5.3
- Roland, N. et al. (2024, March 27). Use of progestogens and the risk of intracranial meningioma: national case-control study. Retrieved from https://www.bmj.com/content/384/bmj-2023-078078.long
- Sample, I. (2024, March 27). Hormone medication could increase risk of brain tumours, French study finds. Retrieved from https://www.theguardian.com/society/2024/mar/27/hormone-medication-brain-tumours-risk-progestogens-study
- Penn Medicine. (n.d.) Meningioma: What is Meningioma? Retrieved from https://www.pennmedicine.org/for-patients-and-visitors/patient-information/conditions-treated-a-to-z/meningioma
Calling this number connects you with a Consumer Notice, LLC representative. We will direct you to one of our trusted legal partners for a free case review.
Consumer Notice, LLC's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
866-934-1922
