Inferior Vena Cava (IVC) Filters
IVC filters are small, cage-like devices placed in the inferior vena cava — the largest vein in the body — to prevent blood clots from reaching the lungs and causing a pulmonary embolism. Due to potential complications with the devices, the U.S. Food and Drug Administration has recommended that IVC filters be removed 29 to 54 days after they’re implanted.
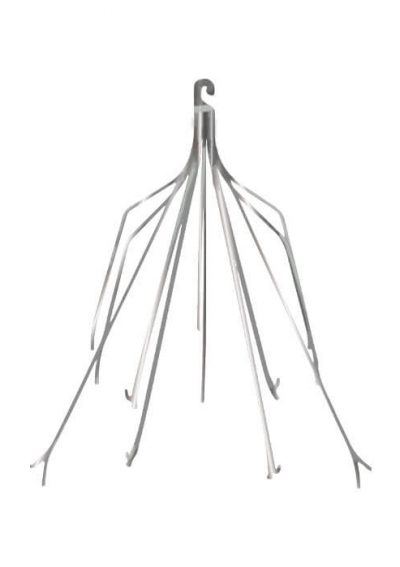
Why Are IVC Filters Used?
A doctor may implant an IVC filter in you to stop blood clots following major surgery or if you have been in a traumatic accident that has caused blood loss. The devices are used in situations in which blood thinners would not be safe or effective.
IVC filters were originally intended only for patients with a risk of deep vein thrombosis or who could not take anticoagulants. But the introduction of retrievable IVC filters in the early 1990s led to the use of these devices in patients with other conditions. By 2016, the devices were used in a variety of treatments, including as a safety measure for people who had suffered multiple traumas or spinal cord injuries. They are also now used in combination with blood thinners, according to the American College of Cardiology.
IVC filters get their name from the vein in which they are implanted — the inferior vena cava, or IVC. It’s a large vein that runs through the lower and middle body. After blood delivers oxygen to the lower body, it flows back up the inferior vena cava and into the heart before picking up more oxygen from the lungs.
Reasons People Receive IVC Filters
- Diagnosed with deep vein thrombosis
- Diagnosed with pulmonary embolism
- Physically unable to move
- Suffering from physical trauma
Events such as major surgery or traumatic accidents that cause blood loss trigger the body’s blood clotting response. This can cause a condition called deep vein thrombosis, or DVT. DVT is a blood clot that forms in the veins of the lower leg or thigh.
Symptoms of Deep Vein Thrombosis
- Warmth or tenderness over the vein
- Pain or swelling in the lower leg or thigh
- Skin redness
When these blood clots break free, they can travel to the lungs and cause a blockage called a pulmonary embolism, or PE. Pulmonary embolisms can cause permanent lung damage or low oxygen levels in your blood that can lead to damage in other organs.
How Does an IVC Filter Work?
IVC filters have long, wiry legs called struts that extend to the walls of the inferior vena cava. Hook-shaped bends at the ends of these struts hold onto the vein wall. Blood can flow through the filters, but blood clots are too large and get trapped.
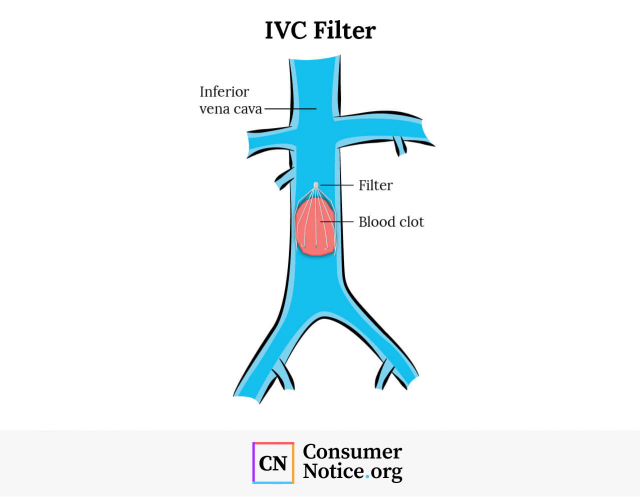
Once a blood clot is caught in an IVC filter, it’s trapped and dissolves naturally until it’s gone or too small to be a threat to the lungs.
Types
These blood clot filters come in retrievable and permanent models.
Permanent types are used for people with chronic clotting conditions who cannot take blood thinners or use other treatments.
The retrievable versions are designed to be removed after the threat of blood clots has passed. They are often used in people with a short term risk of blood clots. But thousands of these have been left in patients for years.
Retrievable IVC filters may break or move around the body if left in too long. They have been blamed for serious internal injuries and deaths when they fail.
The U.S. Food and Drug Administration recommends that retrievable filters be removed as soon as the risk of blood clotting is over.
Placement and Removal
IVC filters are placed and removed by specialized doctors called interventional radiologists. They specialize in minimally invasive surgery that relies on imaging devices such as x-rays or ultrasounds to guide their procedures.
The radiologist will make a small incision in your upper leg or neck and then insert a long, flexible tube called a catheter. The doctor uses medical imaging devices to watch the route of the catheter as he or she gently and carefully snakes it through your blood vessels until it reaches the inferior vena cava.
Once the catheter is in position, the radiologist sends a collapsed IVC filer through the catheter using a specialized tool. After it passes through the open end of the catheter into the inferior vena cava, the filter’s struts deploy and it is implanted in the vein. The radiologist then carefully withdraws the catheter and closes the incision.
Removing an IVC filter is a similar procedure. A radiologist will again use a catheter and special tool to collapse the filter, withdrawing it through the catheter. But if the filter breaks, moves inside the body or becomes embedded in the vein wall before your removal procedure is scheduled, you may require advanced filter retrieval.
An example is the use of lasers to gently shave away layers of scar tissue that have embedded the device’s struts into the vein wall until it can be removed.
What to Expect in the Procedure
The procedure generally takes 30 minutes, but could take up to 90 minutes if more complicated extraction techniques are needed.
In most cases, you will be given a local anesthetic where the doctor will make the incision, but you will most likely be awake for the procedure. You shouldn’t feel much more than pressure around the incision as the catheter is inserted and later removed.
You should be able to resume normal activities in 24 hours.
How to Prepare for IVC Filter Placement
Talk with your doctor before each procedure about steps you should take before IVC filter placement and removal.
Have a list of all your medications and go over them with your doctor in meetings before the surgery. He or she may want to change or stop certain drugs that could cause problems with the procedure. Don’t stop taking any medicine unless your doctor tells you to.
You should not eat anything after midnight the night before IVC filter placement or removal. Drink no more than 12 ounces of water between midnight and 2 hours before the surgery. And don’t eat or drink anything, including water, in the final two hours before the procedure.
Do’s and Don’ts for the Day of IVC Filter Placement or Removal
- Don’t apply lotions, creams or make-up
- Don’t wear contact lenses to the procedure
- Remove all jewelry including piercings and leave them at home with other valuables
- Take only the medications your doctor tells you to on the day of surgery
- Wear loose, comfortable clothing
What to Expect During Recovery from Placement or Removal
You may spend several hours in a recovery room after your procedure.
You should leave the bandage over your incision for 24 hours. Also wait at least 24 hours before showering or otherwise getting the incision wet.
The incision site may feel sore for up to two days. You should be able to treat any pain with over-the-counter pain medicine.
Avoid certain movements and activities — especially those that involve stretching — for the first few days. Your doctor should go over a list of activities to avoid.
When to Call Your Doctor Following IVC Filter Placement or Removal
- Bleeding around the incision
- Chest pain
- Coldness or numbness in your arms or legs
- Continued headache or nausea
- Drainage around incision site
- Fever of 100.4 degrees or higher
- Pain around the incision that doesn’t go away
- Redness and swelling around the incision
IVC filters will not set off metal detectors and they are safe if you require an MRI. But you should let the doctors and technicians at imaging centers know that you have an IVC filter if you do go in for a scan.
Complications
IVC filter complications can occur during placement, after the devices have been in the body for a while or during their removal.
But more severe complications can cause long-term injuries or death, and may make it difficult or impossible to remove the devices.
Complications during filter placement are usually minor and most can be easily treated.
IVC Filter Placement Complications
- Incision site complications
- Minor complications include bleeding, inadvertent punctures of arteries and infection. Less than one percent of these turn into major complications.
- Malposition
- IVC filters may sometimes be placed in faulty positions. This can happen when the filter is placed on the wrong side of where the renal vein connects to the IVC, failing to maximize the blood clot-trapping qualities of the filter. Or it may be placed in the wrong vein altogether.
- Defective filter deployment
- This can happen if the IVC filter’s struts become tangled or fail to properly expand and take hold in the vein.
Delayed complications are among the most serious IVC filter side effects. They can result in severe injuries and, in rare cases, can cause death.
Delayed Complications
- Migration
- IVC filters can migrate from where they were originally placed to other parts of the inferior vena cava or into the heart. It can be dangerous or even deadly to attempt to retrieve or remove an IVC filter once it enters the heart and may require major surgery.
- Deep vein thrombosis
- Though IVC filters are meant to prevent blood clots from DVT from reaching the lung, there has been some research that suggests the devices may increase the risk of developing DVT in patients who never had it before having a filter implanted.
- Filter fracture
- This involves struts breaking off the device and usually occurs if the device has been in place for more than a year. This happens in about 1 to 2 percent of filters in long-term use. It’s also the most common complication reported with retrievable IVC filters. The broken struts have been reported to have traveled to blood vessels serving the lungs, renal veins and into the heart.
- IVC perforation
- IVC filters can sometimes perforate the wall of the inferior vena cava as soon as they are deployed or as a delayed consequence. A 2016 study in Cardiovascular Diagnosis & Therapy reported that 20 percent of IVC filter complications in the U.S. Food and Drug Administration’s MAUDE database were of vein perforations. Patients usually don’t experience symptoms when this happens, unless the struts also perforate organs or other structures outside the IVC. These can damage the duodenum, the upper part of the small intestine, as well as perforate the aorta — the body’s largest artery which carries blood away from the heart.
IVC filter removal complications vary widely and are often associated with different filter types or models. Severe complications such as perforation, migration and organ damage have been named in IVC filter lawsuits against the makers of specific brands of the devices.
The longer a filter is implanted, the higher the chances are for complications during removal.
If the filter has tilted or become embedded in the vein, it can make removal difficult. This means the procedure may take longer than expected or require improvised retrieval techniques.
Removing retrievable IVC filters as soon as the danger of DVT has passed is perhaps the single best way to prevent later removal complications.
Recalls
Manufacturers recalled almost 145,000 IVC filters between 2005 and 2019 in just five major recalls. Manufacturers have seldom recalled the devices despite complications reported to the U.S. Food and Drug Administration.
Cook Medical Gunther Tulip IVC Filter
Cook Medical recalled more than 91,000 Gunther Tulip IVC filters in Feb. 2019. The company reported an error in the instructions for use shipped with the recalled filters.
Cook reissued new instructions for the filters.
Boston Scientific Greenfield IVC Filter
Boston Scientific issued two recalls for its Greenfield IVC filters in 2005.
In Dec. 2005, the company recalled 18,000 filters due to a defect that allowed the carrier capsule to detach while the filter was being implanted. The recall notice said if that happened, it could cause an embolism in the heart or lungs. The FDA declared it a Class I recall, the agency’s most serious kind.
The company had issued another Greenfield IVC filter recall in Aug. 2005, for another issue. In this case, two lots of the filters had a defect in the delivery system. It lacked a critical taper in the design. The recall notice warned that it could tear the vein during implantation.
Bard Denali IVC Filter
Bard recalled 1,183 Denali IVC filters in March 2015. The devices were shipped without certain warnings in their labels. The labels were supposed to tell doctors that the devices should not be used in patients with uncontrolled sepsis or with known nickel-titanium alloy hypersensitivity.
The devices did not have to be returned to the company. Bard notified health care providers who had purchased the affected filters of the problem and told them that, if the filters did not meet their needs, the company would send them a replacement filter.
Cordis OptEase IVC Filter
Cordis recalled about 33,000 OptEase IVC filters in March 2013.
The company shipped the devices with an error in the instructions that could result in the filters being implanted upside down. The company sent out a correction.
The FDA declared it a Class I recall which meant there was a “reasonable probability” that the error would cause “serious adverse health consequences or death.”
13 Cited Research Articles
Consumernotice.org adheres to the highest ethical standards for content production and references only credible sources of information, including government reports, interviews with experts, highly regarded nonprofit organizations, peer-reviewed journals, court records and academic organizations. You can learn more about our dedication to relevance, accuracy and transparency by reading our editorial policy.
- Grewal, S., Chamarthy, M.R., and Kalva, S.P. (2016, December). Complications of Inferior Vena Cava Filters. Cardiovascular Diagnosis & Therapy. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5220210/
- Johns Hopkins Medicine. (n.d.). Inferior Vena Cava (IVC) Filter Placement. Retrieved from https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/inferior-vena-cava-ivc-filter-placement
- Lesney, M.S. (2014, June 19). FDA: Remove IVC Filters 29-54 Days After Implantation. Vascular Specialist. Retrieved from https://www.mdedge.com/content/fda-remove-ivc-filters-29-54-days-after-implantation
- Memorial Sloan Kettering Cancer Center. (2019, March 28). About Your Inferior Vena Cava (IVC) Filter Placement. Retrieved from https://www.mskcc.org/cancer-care/patient-education/ivc-filter-placement
- RadiologyInfo.org. (2018, April 30). Inferior Vena Cava Filter Placement and Removal. American College of Radiology and Radiological Society of North America. Retrieved from https://www.radiologyinfo.org/en/info/venacavafilter
- U.S. Food and Drug Administration. (2005, August 5). Class 2 Device Recall The Greenfield Vena Cava Filter. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?id=41033
- U.S. Food and Drug Administration. (2005, December 2). Class 1 Device Recall Greenfield Vena Cava Filter System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?id=43177
- U.S. Food and Drug Administration. (2013, March 29). Class 1 Device Recall OptEase Vena Cava Filter. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=119066
- U.S. Food and Drug Administration. (2015, March 13). Class 2 Device Recall Bard Denali IVC Filter. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=142563
- U.S. Food and Drug Administration. (2019, February 25). Class 3 Device Recall Gunther Tulip Vena Cava Filter Sets. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=171163
- U.S. National Library of Medicine. (2019, November 8). Pulmonary Embolism. Retrieved from https://medlineplus.gov/pulmonaryembolism.html
- U.S. National Library of Medicine. (2019, October 15). Deep Vein Thrombosis. Retrieved from https://medlineplus.gov/deepveinthrombosis.html
- Van Ha, T.G. (2006, June). Complications of Inferior Vena Caval Filters. Seminars in Interventional Radiology. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036364/
Calling this number connects you with a Consumer Notice, LLC representative. We will direct you to one of our trusted legal partners for a free case review.
Consumer Notice, LLC's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
877-537-0150

