Paragard
Paragard, the only hormone-free IUD in the U.S., provides long-term birth control with copper as its active ingredient. Effective for up to 10 years, it may cause side effects like heavy periods and backaches. Breakage during Paragard removal has led to lawsuits and FDA scrutiny.
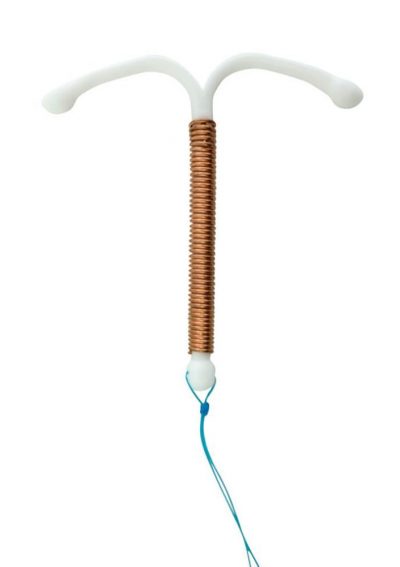
What Is the Paragard IUD?
Paragard is the only hormone-free IUD available in the United States, and it has been on the market since 1984. According to Cooper Surgical, the IUD’s manufacturer, Paragard has been proven safe and effective since its release.
Cooper markets the medical device as a convenient, no-hassle birth control device that is over 99 percent effective. Once in place, Paragard works for up to 10 years and can be removed whenever the user wishes to conceive.
Most insurance plans cover Paragard, which is available at little to no cost out of pocket.
How Does Paragard Work?
Paragard is a small, flexible, T-shaped plastic device with copper wrapped around it. The implant’s stem is about 1.42 inches tall, and its arms measure about 1.26 inches across. Strings are attached to it for easy removal.
Unlike other IUDs, such as Skyla and Mirena, Paragard doesn’t contain hormones. It doesn’t prevent ovulation or stop periods. Instead, it uses copper to interfere with sperm mobility, so they can’t fertilize the egg.
Paragard works to prevent pregnancy as soon as it’s in place.
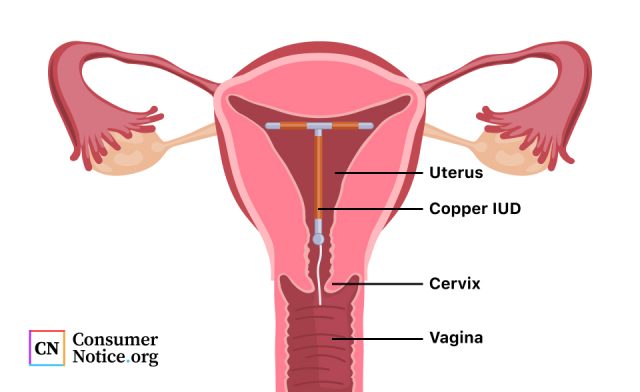
Paragard Insertion and Removal
Paragard insertion and removal are outpatient procedures that don’t require surgery or general anesthesia. Some providers may use a local anesthetic.
Each procedure takes only a few minutes in a medical provider’s office or clinic. Patients can usually go home a few minutes after implantation or removal.
Insertion
After an examination to determine the position of the uterus, the medical provider will clean the vagina and cervix and measure the uterus.
The provider will then load the Paragard into a plastic tube and insert it into the uterus. The IUD has flexible arms that extend once it’s deployed. The provider will then remove the tube, leaving the IUD in place.
The provider trims the threads so they extend slightly out of the cervix and long enough for a patient to feel them during a self-check.
Some people may experience discomfort during the insertion of an IUD. This includes cramping, pinching, dizziness, nausea and feeling faint. Most symptoms go away quickly after insertion, but cramping can last a few hours to a few days.
Removal
Paragard removal takes only a few minutes in a medical provider’s office. The provider grasps the exposed threads with an instrument and gently pulls downward. The Paragard’s arms fold up during removal, allowing it to come out of the uterus.
Some people may feel discomfort during removal, but it only lasts a few minutes. According to the prescribing information, bleeding, dizziness, slow heartbeat or rare seizures may occur during removal (or insertion). Rarely, Paragard may become stuck, and surgery may be required to remove it.
Because of the possibility of complications, Paragard should be removed by a medical provider.
Complications and Side Effects
Because Paragard uses copper as its active ingredient instead of hormones, it doesn’t cause weight gain. Like most IUDs, Paragard is generally safe for most people, but it has a few potential side effects.
- Anemia
- Backache
- Cramping
- Heavier or longer than normal periods
- Pain
- Pain during sex
- Painful periods
- Partial or complete expulsion
- Spotting in between periods
- Vaginitis
Serious Side Effects
Common side effects with Paragard are usually mild. But rarely, serious problems can occur. These include pregnancy outside of the uterus (ectopic pregnancy), severe infections, septic abortion, perforation or embedment in the uterus and pelvic inflammatory disease.
Paragard isn’t for everyone. Don’t use Paragard if you have an active infection, suspect you have cancer, have pelvic inflammatory disease or are allergic to copper or other components of the IUD.
While Paragard is made of copper, there has been no sufficient evidence to suggest it causes copper poisoning or toxicity.
FDA Actions on Paragard
In June 2024, the U.S. Food and Drug Administration updated the information about Paragard to provide more details about the device’s potential to break. This update came after the FDA’s thorough safety review in 2021.
“Partial penetration or embedment of Paragard in the myometrium can make removal difficult; surgical removal may be necessary. Breakage of an embedded Paragard during non-surgical removal has been reported.”
The FDA is still monitoring Paragard’s safety, as it does for all products it approves. In the information they released, FDA safety officials acknowledged that if parts of the IUD break off and remain inside the body, there could be “unknown consequences.” They also noted that “device breakage can subject patients to risk and cause anxiety for both patients and healthcare providers.”
As of November 2024, there has been a significant rise in reports about Paragard breaking, with over 7,000 such reports submitted to the FDA’s system for tracking adverse events.
Lawsuits
As of December 2025, there were 3,749 Paragard lawsuits pending in a multidistrict litigation in a Georgia federal court.
People have filed Paragard lawsuits because the IUD can break during removal and cause complications such as pain, perforation of the uterus or cervix and infertility. Some of these plaintiffs required surgery to remove the broken pieces.
Plaintiffs allege Cooper Surgical failed to warn them about the risk of complications from Paragard breakage during removal. The lawsuits also claim Paragard is defective.
In December 2020, the Judicial Panel on Multidistrict Litigation, under Judge Leigh Martin May, consolidated several lawsuits from across the country in the Northern District of Georgia.
Case Study: Complications Associated with Paragard IUD Removal
Jennifer Lewis, a Michigan resident, opted for a Paragard IUD as her contraceptive choice. The device was marketed as a safe, reversible and straightforward option for birth control. However, during its removal in 2021, complications arose, culminating in a stressful and painful experience.
- Discovering Paragard Breakage
- While undergoing an unrelated surgery, doctors discovered that the Paragard had broken inside her uterus. This necessitated additional procedures to retrieve the fragments, with the risk of a hysterectomy looming had the pieces not been removed promptly. Lewis endured heightened pain and suffering, along with significant medical expenses.
- Lewis Takes Legal Action
- Lewis filed a lawsuit against multiple manufacturers, citing design and manufacturing defects, failure to warn and negligence. Her case underscores broader concerns about the risks of breakage during Paragard removal, challenging its claims of easy reversibility.
Editor Lindsay Donaldson contributed to this article.
11 Cited Research Articles
Consumernotice.org adheres to the highest ethical standards for content production and references only credible sources of information, including government reports, interviews with experts, highly regarded nonprofit organizations, peer-reviewed journals, court records and academic organizations. You can learn more about our dedication to relevance, accuracy and transparency by reading our editorial policy.
- Moreschi, A., et al. (2024, November 14). FDA Launched Safety Review Into Broken IUDs After Spotlight Inquiry, but Questions Remain. Retrieved from https://cbsaustin.com/news/spotlight-on-america/spotlight-inquiry-led-to-fda-safety-review-of-paragard-breakage-but-questions-remain
- U.S. Judicial Panel on Multidistrict Litigation. (2024, November 1). MDL Statistics Report - Distribution of Pending MDL Dockets by District. Retrieved from https://www.jpml.uscourts.gov/sites/jpml/files/Pending_MDL_Dockets_By_Actions_Pending-November-1-2024_0.pdf
- Cooper Surgical. (2024, February). What Is Paragard? Retrieved from https://www.paragard.com/what-is-paragard/
- U.S. District Court for the Northern District of Georgia, Atlanta Division. (2022, September 28). Lewis v. Teva Pharmaceuticals USA, Inc., et al. Retrieved from https://www.pacer.gov
- United States Judicial Panel on Multidistrict Litigation. (2020, December 16). Transfer Order. Retrieved from https://www.jpml.uscourts.gov/sites/jpml/files/MDL-2974-Transfer%20Order-12-20.pdf
- Cooper Surgical. (2020, February). Paragard Prescribing Information. Retrieved from https://hcp.paragard.com/wp-content/uploads/2018/09/PARAGARD-PI.pdf
- U.S. Food and Drug Administration. (2005, September). Proposed Prescribing Data Paragard. Retrieved from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf
- CooperSurgical. (n.d.). Paragard; Important Safety Information. Retrieved from https://www.coopersurgical.com/product/paragard/
- Planned Parenthood. (n.d.). How Much Does An IUD Cost? Retrieved from https://www.plannedparenthood.org/learn/birth-control/iud/how-can-i-get-an-iud
- Planned Parenthood. (n.d.). What’s An IUD Insertion Like? Retrieved from: https://www.plannedparenthood.org/learn/birth-control/iud/whats-an-iud-insertion-like
- United States District Court Northern District of Georgia. (n.d.). In re: Paragard IUD Products Liability Litigation. Retrieved from: https://www.gand.uscourts.gov/20md2974
Calling this number connects you with a Consumer Notice, LLC representative. We will direct you to one of our trusted legal partners for a free case review.
Consumer Notice, LLC's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
844-891-3125

