IVC Filter Lawsuits
Lawsuits claiming IVC filters caused damage or death are ongoing, including more than 7,700 pending cases against Cook Medical. Plaintiffs have received major settlements for cases against C.R. Bard and Rex Medical, while a lawsuit filed in 2016 against C.R. Bard is moving toward trial.
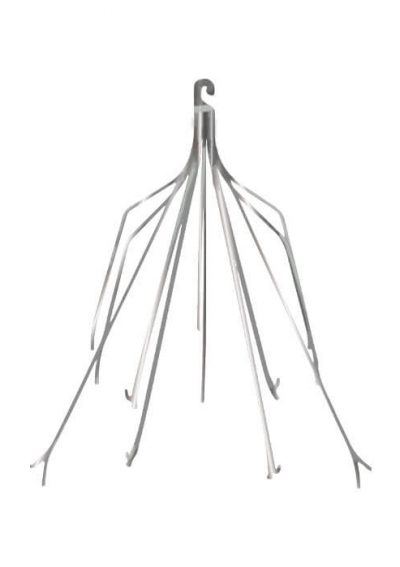
Latest Updates in IVC Filter Lawsuits
As of April 2025, inferior vena cava filter multidistrict litigation against C.R. Bard was no longer open following confidential settlements. Individual lawsuits continue to be filed and Maria Dalbotten’s case filed against C.R. Bard in 2016 is currently advancing toward trial.
There were still 7,202 pending cases in MDL 2570 against Cook Medical before Senior Judge Richard L. Young of the U.S. District Court for the Southern District of Indiana. Individual lawsuits against IVC filter manufacturers ALN, Argon, Boston Scientific, Cordis and Rex Medical were also in progress.
- November 2023: Attorneys continued filing cases in the open Cook Medical MDL for people who experienced injuries. While the C.R. Bard MDL is closed, plaintiffs are still filing individual cases.
- August 2023: On Aug.11, U.S. Court of Appeals for the Seventh Circuit decided in favor of plaintiff Natalie Johnson, upholding a $3.3 million verdict against C.R. Bard.
- July 2021: Jurors in Dallas awarded plaintiff Debra Branch $386,250 in damages in her lawsuit against C.R. Bard, after her IVC filter fractured, causing numerous complications.
- June 2021: After her filter changed position and punctured a vein, resulting in several surgeries to repair and remove it, a Wisconsin jury awarded Natalie Johnson a $3.3 million verdict in her lawsuit against C.R. Bard.
- May 2021: Justin Peterson from Portland, Oregon, who required major abdominal surgery after a filter perforated his vena cava, was awarded $926,000 in his lawsuit against C.R. Bard.
- August 2020: Courts awarded Sherr-Una Booker $3.6 million in her lawsuit against C.R. Bard. The award included $2 million in punitive damages and $1.6 million in compensatory damages.
IVC filters are medical devices implanted in patients’ inferior vena cava to reduce the risk of serious blood clots. Implantation usually occurs after surgery to remove clots. Doctors may also use them when patients are at risk of clots but can’t take blood thinners. If these cage-like devices break or move in the body, they can cause damage to internal tissue and organs or even death.
Why Are People Filing IVC Filter Lawsuits?
People are filing IVC filter lawsuits because implants to prevent embolisms migrated or broke. People are also filing lawsuits for complications resulting from the procedure required to remove the device. More than 7% of patients required two or more removal attempts.
The bulk of these lawsuits are product liability cases. The defendants in these cases are the manufacturers of the filters rather than medical professionals as in malpractice cases.
- Complications stemming from removal or attempted removal of IVC filter
- Components detached from the filter and remained in the body
- Death
- Filter fractured or broke
- Filter migrated
- Filter perforated an organ or vein
Recent medical research has raised concerns about the use of IVC filters, as there is no demonstrable decrease in mortality with their use. However, a recent study demonstrates that the results of filter removal procedures improve dramatically when the implanting doctors are active in their follow-ups with patients.
IVC Filter FDA Response and Recall
In July 2015, the FDA issued a formal warning letter to C.R. Bard concerning violations found at two Bard facilities. These violations included misbranding and quality system regulations infractions. It also accused the company of manufacturing and selling non-approved medical devices.
In 2010 the FDA issued a communication advocating for the removal of IVC filters when no longer necessary, optimally 29-54 days after the original procedure. In 2014, the FDA updated their communication, reemphasizing the importance of removing IVC filters in the appropriate time frame. The FDA has issued a small number of recalls of IVC products for different reasons.
- Gunther Tulip: In 2019, the manufacturer recalled 91,731 units of Cook Medical’s Gunther Tulip to update usage instructions.
- Bard Denali: In 2015, there was a recall of 1,183 units of C.R. Bard’s Denali filters because the labels required a change of information.
- Cordis Optease: A printing error led to a recall of 33,000 units of Cordis’ OPTEASE filter in 2013. This error could cause an upside-down installation of the unit, which would necessitate a second procedure to correct the problem.
- Greenfield: A recall began in 2005 for 18,000 units of Boston Scientific’s Greenfield filter because of the risk of components detaching and potentially causing embolisms.
A recall isn’t necessary to file a lawsuit for damage or injury. For many of the lawsuits, the filers were patients whose IVC filters remained in place long after the advised 29–54-day period. These patients had no idea the doctors should have removed the filters.
Can I File an IVC Filter Lawsuit?
You may be eligible to file a lawsuit if your IVC filter fractured, if the whole device or pieces of it migrated, if it perforated your organs or tissue or if removal of the filter caused complications. The families of patients who died from these issues may also be eligible.
A successful lawsuit can compensate you for emotional, financial and physical hardships. Compensation can also help you recover lost wages and cover further medical treatment. It’s crucial to speak with an experienced attorney who can assess your case.
IVC Filter Settlements and Verdicts
C.R. Bard has negotiated both settlements in individual lawsuits and a global settlement for multidistrict litigation with more than 8,000 plaintiffs. Cook Medical chose the trial route, winning the first bellwether lawsuits but losing subsequent trials. Currently there are more than 7,900 cases in the multidistrict litigation docket against Cook.
- June 2021: A Wisconsin plaintiff received a $3.3 million verdict against C.R. Bard.
- October 2019: Courts awarded Tracy Reed-Brown $33.7 million in her suit against Rex Medical. This is the largest settlement to date and includes punitive damages of $30,315,726.
- July 2018: A Texas state (rather than federal) lawsuit awarded a former firefighter $1.2 million in their lawsuit against Cook Medical.
Legal experts suggest the average amount awarded in an IVC settlement will vary. Factors affecting settlement amounts include the nature of the injury, the manufacturer, and the inclusion of punitive damages.
14 Cited Research Articles
Consumernotice.org adheres to the highest ethical standards for content production and references only credible sources of information, including government reports, interviews with experts, highly regarded nonprofit organizations, peer-reviewed journals, court records and academic organizations. You can learn more about our dedication to relevance, accuracy and transparency by reading our editorial policy.
- United States Judicial Panel on Multidistrict Litigation. (2025, April 1). MDL Statistics Report - Distribution of Pending MDL Dockets by Actions Pending. Retrieved from https://www.jpml.uscourts.gov/sites/jpml/files/Pending_MDL_Dockets_By_Actions_Pending-April-1-2025.pdf
- Sterbis, E. et al. (2023, March 16). Inferior Vena Cava Filter Retrieval Rates Associated With Passive and Active Surveillance Strategies Adopted by Implanting Physicians. Retrieved from https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2802524
- Johnson, M.S. et al. (2023, February 23). Predicting the Safety and Effectiveness of Inferior Vena Cava Filters (PRESERVE): Outcomes at 12 months. Retrieved from https://www.jvir.org/article/S1051-0443(22)01406-3/fulltext
- Steinberg, J. (2023, January 12). Bard Blood-Clot Filter Injury Claims Advance Towards Trial. Retrieved from https://news.bloomberglaw.com/litigation/bard-blood-clot-filter-injury-claims-advance-toward-trial
- Muneeb, A. & Dhamoon, A. (2022, August 9). Inferior Vena Cava Filter. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK549900/
- Ahmad, Y. et al. (2022, July 18). Inferior Vena Cava Filter Litigation Review: An Analysis of Medicolegal Cases Pertaining to Inferior Vena Cava Filters. Retrieved from https://www.jvir.org/article/S1051-0443(22)01066-1/fulltext
- Ho, K.M. et al. (2022, May 26). A prospective ex vivo biomechanical analysis of retrievable inferior vena cava filters. Retrieved from https://www.jvsvenous.org/article/S2213-333X(22)00242-6/fulltext
- Russel, J. (2022, February 25). Cook Medical legal battle one of largest in state history. Retrieved from https://www.ibj.com/articles/cook-medical-legal-battle-is-one-of-largest-in-state-history
- U.S. Food & Drug Administration. (2021, December 21). FDA Grants Marketing Authorization for Inferior Vena Cava Filter Removal Device. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-grants-marketing-authorization-inferior-vena-cava-filter-removal-device
- Wolf, M. (2021, July 26). Dallas law firm's combined verdicts top 7 million in series of blood clot filter lawsuits. Retrieved from https://www.dallasnews.com/business/local-companies/2021/07/26/dallas-law-firms-combined-verdicts-top-7-million-in-series-of-blood-clot-filter-lawsuits/
- Bikdeli, B. et al. (2018, December 10). Association of Inferior Vena Cava Filter Use With Mortality Rates in Older Adults With Acute Pulmonary Embolism. Retrieved from https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2717952
- FDA. (2015, July 13). Warning Letter C.R. Bard, Inc. Retrieved from https://web.archive.org/web/20200811220730/https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/cr-bard-inc-453127-07132015
- FDA. (2014, May 6). Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication. Retrieved from http://web.archive.org/web/20150806071825/http://www.fda.gov:80/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm
- John Hopkins Medicine. (n.d.). Inferior Vena Cava (IVC) Filter Placement. Retrieved from https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/inferior-vena-cava-ivc-filter-placement
Calling this number connects you with a Consumer Notice, LLC representative. We will direct you to one of our trusted legal partners for a free case review.
Consumer Notice, LLC's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
877-537-0150
