Exactech Files for Bankruptcy in Wake of Mounting Lawsuits
Editors carefully fact-check all Consumer Notice, LLC content for accuracy and quality.
Consumer Notice, LLC has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
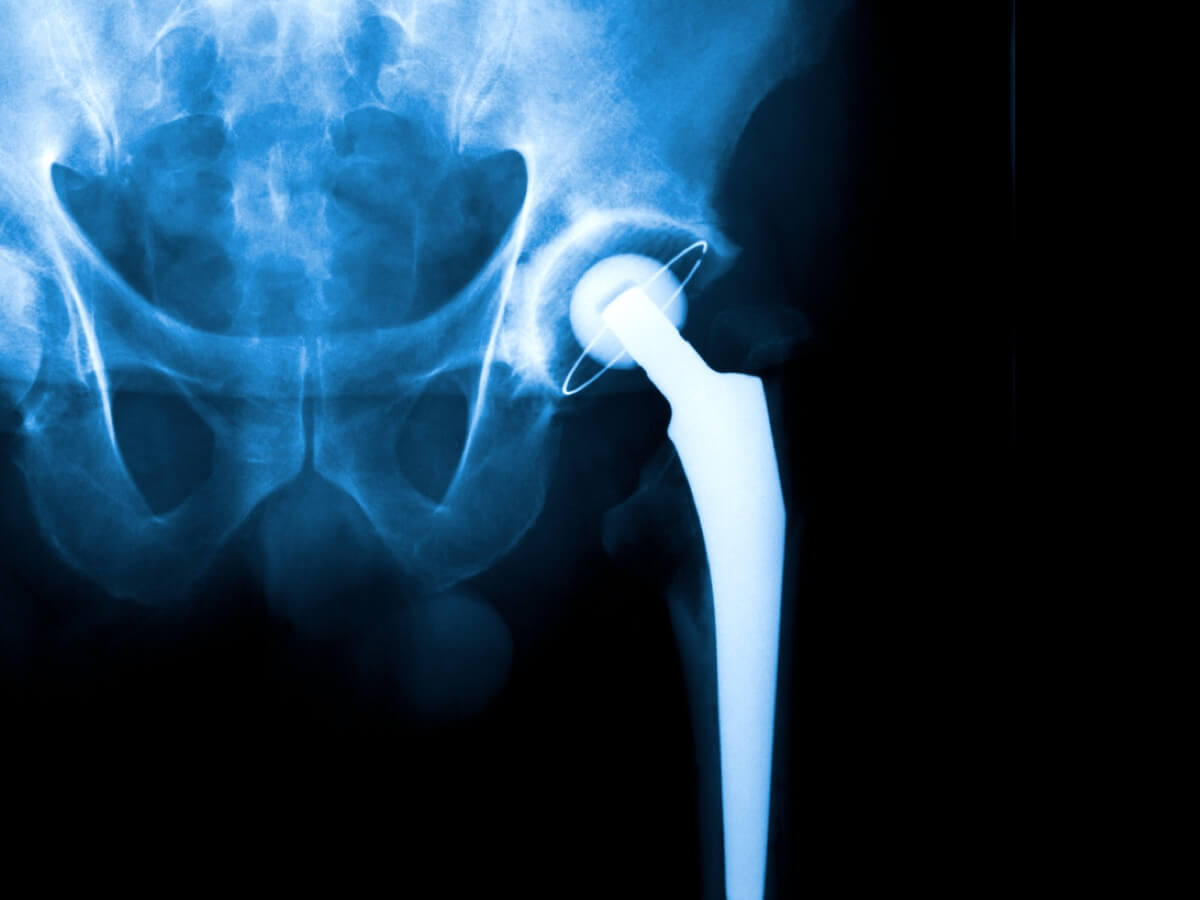
Exactech has filed for bankruptcy, casting doubt on the future direction of the hundreds of active lawsuits against the medical technology company.
The Florida-based hip and knee replacement manufacturer credited the decision to file for Chapter 11 to the mounting claims that defective packaging led to early wear and device failures. There were 1,770 Exactech lawsuits pending in multidistrict litigation this month, with nearly 600 new lawsuits being filed so far this year.
“We face unsustainable liabilities associated with knee and hip litigation related to the packaging recalls we voluntarily initiated between 2021 and 2022,” President and CEO Darin Johnson said in a statement. “We take our commitment to patient well-being very seriously and have provided substantial out-of-pocket patient reimbursements and surgeon support for related expenses.”
According to CBS, the progress of those lawsuits has been paused for now as part of the bankruptcy filing. The MDL had been moving along, with new dates for two bellwether trials announced just last week.
Those trials, which were set to begin in September and November of next year, will likely now see further delays with litigation on hold.
Exactech said that it plans to sell its assets to a group of existing investors. Those investors will also contribute $85 million to fund the company’s operations during the bankruptcy proceedings.
Exactech Lawsuits Blame Early Wear, Device Failures on Defective Packaging
Exactech’s troubles first began in 2021, when the company decided to recall several types of its hip replacement devices due to early wearing. According to the Food and Drug Administration, the cause of that wearing was unknown at the time.
Multiple recalls followed from there, eventually expanding to knee and ankle devices as well. It was discovered that many of Exactech’s products had been packaged in bags that were missing a key layer designed to prevent oxidation.
Without that layer, the devices could be exposed to too much oxygen, resulting in premature wearing and even device failure.
These devices typically last 15 to 20 years, but a KFF News analysis found that surgeons had removed 200 devices in less than seven years.
Hundreds of lawsuits followed, with patients who had been implanted with Exactech devices blaming early wear and failures on the defective packaging issues.
Devices that were implanted in patients between 2004 and 2021 have been included in the recalls. The FDA has warned that some of the recalled devices are associated with bone loss and the need for revision surgery.