Lawyers Select 24 Bard PowerPort Lawsuits for Early Trials in MDL
Editors carefully fact-check all Consumer Notice, LLC content for accuracy and quality.
Consumer Notice, LLC has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
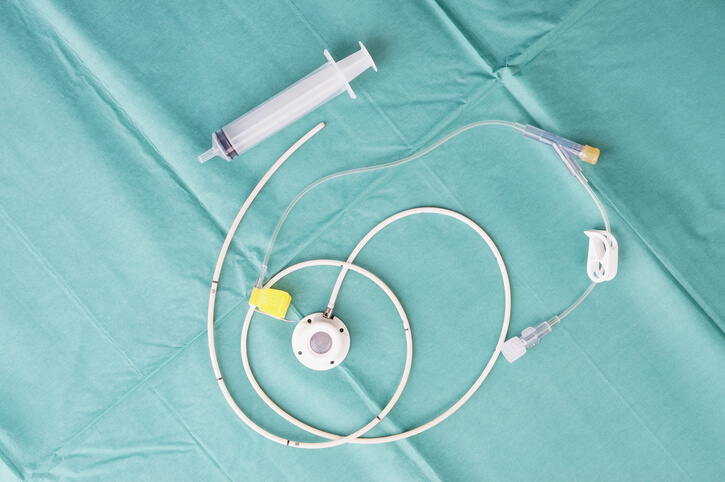
Lawyers in the Bard PowerPort MDL have chosen 24 lawsuits to undergo a fact-finding process in preparation for potential bellwether trials.
These trials aim to gauge jury response to evidence and shape settlements for the over 320 similar cases consolidated in federal court.
Bellwether trials involve a small group of cases selected for early trials. They offer valuable insights into how juries might respond to the broader litigation. By analyzing these trials, both sides can assess their arguments’ strengths and weaknesses, potentially leading to settlement discussions.
Allegations in the Bard PowerPort lawsuits suggest that Bard’s design of the totally implantable vascular access devices (TIVAD), used for chemotherapy and medication delivery, was defective. This allegedly caused severe infections, fractures and other complications due to material degradation.
Lawyers are still taking Bard PowerPort cases. People implanted with a Bard TIVAD who experienced blood clots, cardiac punctures, necrosis, hemorrhage, infection, torn blood vessels, fluid build-up or severe pain may qualify for a lawsuit. A lawyer can help determine whether an injury qualifies for a case.
Next Steps: Fact-Finding and Discovery
The plaintiffs in the 24 selected Bard PowerPort lawsuits need to submit detailed questionnaires by July 31. This information will likely include personal and medical histories, alleged injuries and the impact of the devices on the plaintiffs’ lives.
After plaintiffs submit these fact sheets, the court will set a schedule to begin depositions and gather the discoveries specific to each case. While it’s still too early to tell how much a Bard Powerport lawsuit could be worth, this phase of the litigation could help determine that.
U.S. District Judge David G. Campbell is expected to issue an order detailing the case management and scheduling for these bellwether trials. Campbell has stated that the next update will come after the August 16 state management conference, in which the involved parties will discuss further steps and issues.
Campbell is the same judge who oversaw another MDL involving Bard’s IVC filters, which are used to reduce blood clots in people who cannot take blood thinners. The IVC cases against Bard have closed following confidential settlements.
What is the Bard PowerPort MDL?
The Bard PowerPort MDL was established in August 2023, which combined the cases under Judge Campbell. The U.S. Food and Drug Administration notified the public of a recall of 178 Bard PowerPort catheters in March 2020. Shortly after the recall, plaintiffs filed lawsuits.
As of July 1, there were 322 cases in the MDL and 47 additional cases pending in the Superior Court of New Jersey, according to a joint memorandum filed July 8. However, the New Jersey cases are facing pushback from the defendant.
On May 17, 2024, the New Jersey state liaison re-filed for multi-county litigation (MCL) designation, but the defendants opposed this on June 21, 2024. They argue that only 10 of these cases involve New Jersey residents, while the rest are from non-residents who chose not to file in the MDL for “strategic reasons,” according to the court document.
The defendants plan to dismiss all non-resident plaintiffs’ complaints on forum non conveniens grounds, meaning another court is more appropriate than the state court. Defendants also intend to dismiss many complaints on the grounds of statute of limitations, because over 75% are time-barred.
A hearing for the New Jersey cases is scheduled for August 15.
Editor Lindsay Donaldson contributed to this article.