MDL Judge Reschedules Exactech Bellwether Trials After New Recall
Editors carefully fact-check all Consumer Notice, LLC content for accuracy and quality.
Consumer Notice, LLC has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
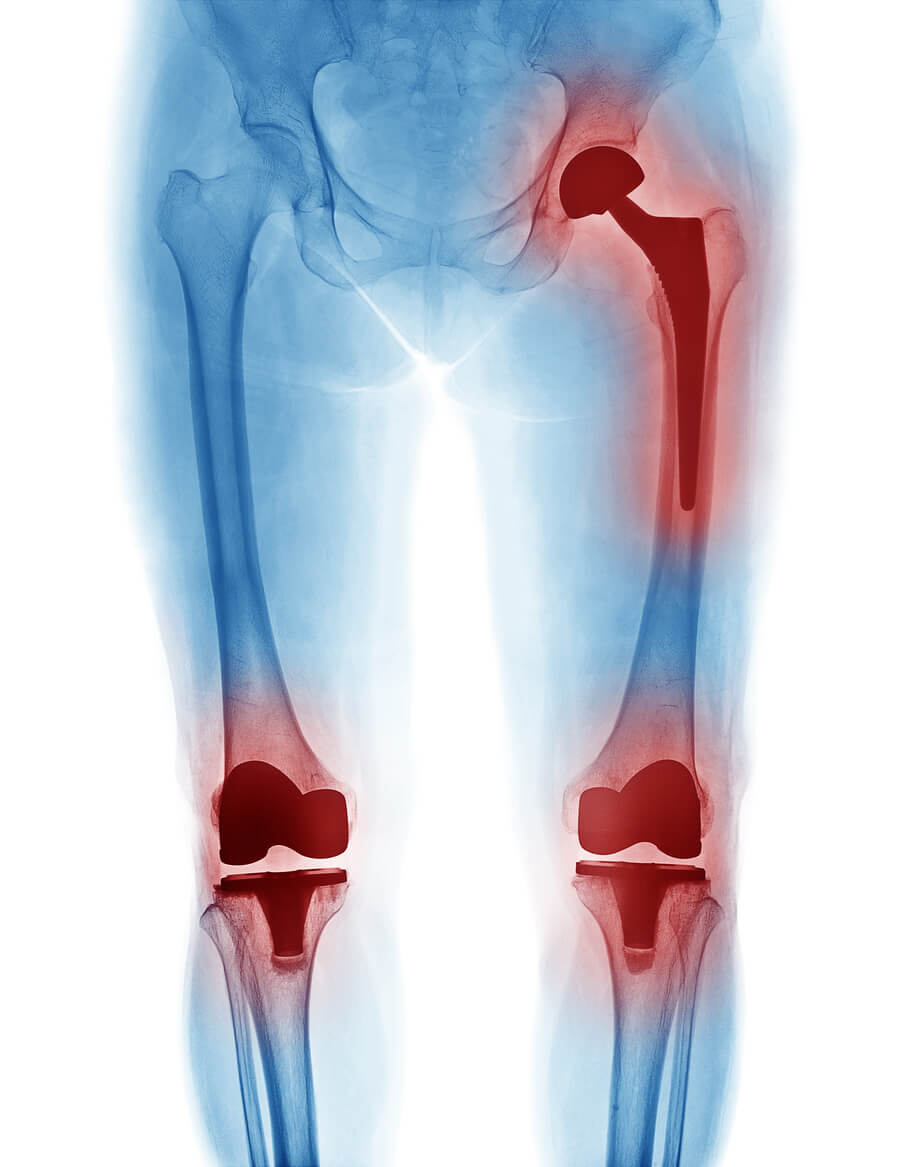
A shake up in the Exactech bellwether trials will allow both sides more time to prepare their cases, after a knee implant recall threw a last-minute wrench into the works.
U.S. District Judge Nicholas G. Garaufis has approved swapping the dates of the first two bellwether trials in the Exactech multidistrict litigation (MDL). The complexity of this legal battle concerning defective knee, hip and ankle implants was further intensified by an Exactech recall in April 2024.
Both plaintiffs and defendants requested the schedule change, citing the importance of additional discovery time in light of the April 2024 knee recall.
The first bellwether trial, Gayle Tarloff’s case, was set for June 2025. However, because the most recent recall involves the patella knee component that affected Tarloff, plaintiffs and lawyers decided they needed more time to prepare for that trial. The court has now prioritized Geraldine Larson’s trial, moving it to June 2025, while Tarloff’s case has been pushed to August 2025.
What This Means for the MDL and Plaintiffs
The Exactech MDL, centralized in the Eastern District of New York, had over 1,600 Exactech lawsuits as of August 2024. These lawsuits focus on claims that Exactech implants were prone to premature failure, resulting in severe injuries such as pain, bone loss and the need for revision surgeries.
The outcomes of the bellwether trials can establish the direction for the remaining cases, potentially influencing settlement discussions and future litigation strategies.
Bellwether trials, often viewed as a litmus test in mass tort cases, help plaintiffs and defendants assess how juries might respond to evidence and legal arguments in similar cases. The stakes are high, as the verdicts could impact hundreds of other pending cases.
Packaging Error at the Root of the Problem
Exactech lawsuits focus on a packaging error that exposed the plastic components in the knee, hip, and ankle implants to excessive oxygen. According to plaintiffs’ complaints, this oxidation caused the implants to degrade prematurely.
Lawsuits claim those injured experienced a range of serious complications, including pain, swelling, bone loss and, ultimately, the need for additional surgeries to replace the failed implants.
The first Exactech recalls began in 2021. However, the issue gained further attention in April 2024 when Exactech expanded the recall to include more knee and shoulder replacement devices.
Editor Lindsay Donaldson contributed to this article.